

current position:Information and data>Oxidation behavior of niobium at high temperature
Niobium is a refractory metal belonging to the VB group. It has high melting point (2468C), low density (7.83g/cm3), good ductility and other characteristics, and can withstand certain mechanical deformation. At the same time, niobium also has relatively stable physical and chemical properties, small thermal neutron capture cross-section (1.15×1024cm2), and excellent corrosion resistance. It is widely recognized and applied in aerospace, nuclear energy industry and medicine.
However, it is easier to be oxidized in the air, which restricts its development in the aerospace field. The low-valence oxides (Nb6O and NbOx) formed below 450°C have certain protective properties [5], and the oxidation rate slows down as the oxidation time increases. When the temperature exceeds 450C, the crystal structure of niobium oxide changes (the unit cell volume changes from 4.36g/cm3 to 5.17g/cm3) and causes cracking and pulverization of the oxide layer. At this time, the protective oxide film transforms to the non-protective oxide film, and the oxidation rate is obviously increased, showing a linear increase, as shown in Figure 1. Generally, pure Nb will have "pest" oxidation phenomenon at 600C. This is because the generated Nb2O5 has a large volume expansion, a large stress is formed on the surface of the oxide layer, and it increases with the increase of the thickness of the oxide layer, thereby reaching a crack The formation and expansion of stress is the root cause of looseness and destruction of the oxide layer, which limits its application [671. Pure sharp's oxidation process is complicated with temperature changes, and it mainly depends on the crystal form transformation of the oxidation product: α-Nb2O5 β-Nb2O5γ-Nb2O5γ'Nb2O5 melting.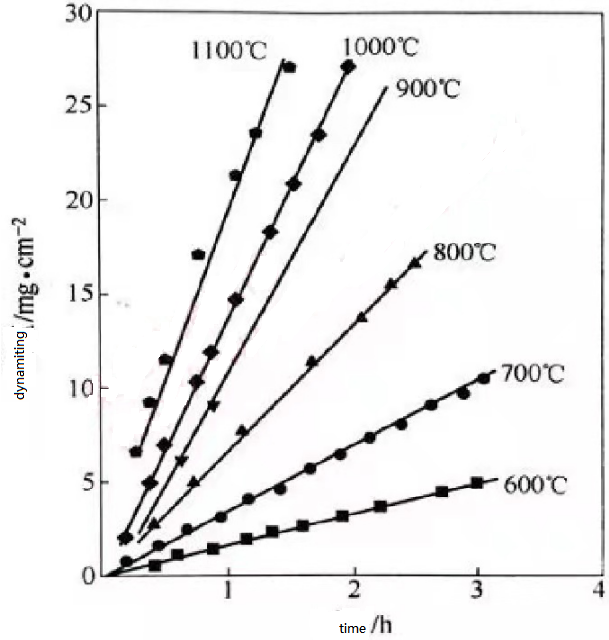
Fig. 1 Oxidation rate of pure Nb in air
Hot information

