

The thermal expansion coefficients of Nd3+ doped LZ powders with different contents from room temperature to 1200°C were investigated, and the results are shown in Figure 1. It can be seen from Figure 1 that the doping of rare earth ions will have a certain impact on the thermal expansion behavior of LZ, and with the increase of Nd3+ content, the thermal expansion coefficient of La2-xNdxZr2O7 complex acid steel powder shows a trend of first decreasing and then increasing. Below 800℃, the thermal expansion coefficient of N04 is the lowest; but when the temperature is higher than 800℃, the thermal expansion coefficient of N06 decreases with the increase of temperature. From room temperature to 1200°C, the thermal expansion coefficient of NO6 is only 8.928×10-6/K, which is significantly lower than that of La2Zr207 (9.694×10-6/K). Coating materials with low coefficient of thermal expansion can be considered for use on low-expansion substrates such as Cf/SiC or Mo alloys. See Chapter 8 for details. Further increasing the doping amount x, the thermal expansion coefficient of La2-xNdxZr2O7 powder began to increase sharply, even exceeding that of pure LZ powder. The thermal expansion coefficient of N08 in the range of 25~1200℃ reached 9.82×10-6/K.
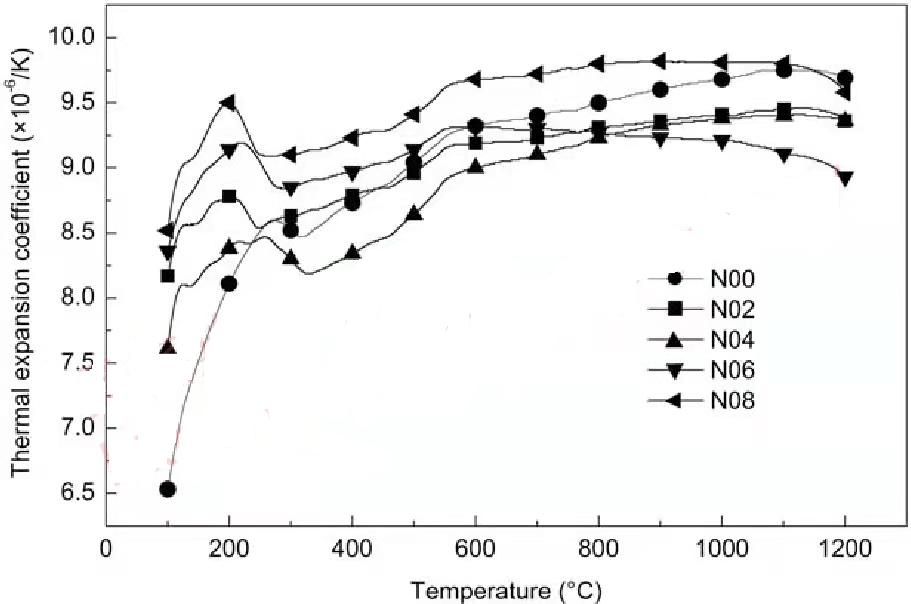
Figure 1 La-xNdxZr2O7 (x=0, 0.2, 0.4, 0.6, 0.8) powder thermal expansion coefficient and temperature relationship curve
The thermal expansion coefficient of a material mainly depends on the repulsive force between atoms in the substance, the size of the gravitational force, and the strength of the chemical bond. The strength of the chemical bond (R-O bond) formed by any ion with oxygen is related to the ionic radius and atomic number of R. The R-O bond energy of light rare earth elements (La~Eu) decreases with the increase of atomic number. For example, the bond energy of La-O bond is 8.25 eV, and the bond energy of Nd-O bond is 7.25 eV. In theory, reducing the strength of the La-O bond is beneficial to the expansion of the La-O bond in the complex acid coating, that is, it is beneficial to increase the thermal expansion coefficient of LZ. However, the experimental results show that the effect of Nd substitution of La on the thermal expansion coefficient is not completely consistent with the changes in ionic radius and bond energy. The reason is not clear, but it is certain that the thermal expansion behavior is not only related to the bond energy.
In addition, it can be seen from Figure 1 that as the temperature increases, the thermal expansion coefficient of the powder material generally shows an increasing trend. The nature of the thermal expansion coefficient of solid materials increasing with increasing temperature is that the average distance between the particles in the lattice structure increases with increasing temperature. After the solid material is heated, the crystal vibration strengthens, which causes the volume to increase. At high temperatures, the intensification of the lattice vibration will increase the thermal expansion coefficient. However, in the temperature range of 200-560°C, a strong shrinkage peak appears on the thermal expansion curve of all materials. This phenomenon has also occurred in La2Ce2O7. Obviously, this shrinkage is not caused by sintering during the thermal expansion test. The reason for the thermal shrinkage of the material is the decrease of the material's own lattice constant. There are several mechanisms that cause the shrinkage of the crystal lattice, including phase transition, asymmetric expansion of the crystal lattice, polyhedral rotation, and shear motion of the M-O-M' (M, M'=metal atom) bond. In the pyrochlore structure La2Zr2O7, there are a large number of oxygen holes, and the longitudinal and shear motion of the M-O-M' bond controls the thermal expansion of the crystal lattice. In a suitable temperature zone, such as 200~560℃, the transverse shearing movement is stronger than the longitudinal movement, which will cause heat shrinkage. After partial Nd3+ ions replace La3+, the oxygen vacancies inside the crystal are increased. Therefore, as the content of Nd3+ increases, the degree of thermal shrinkage of the material is also greater. This kind of thermal shrinkage at lower temperatures will aggravate the thermal stress inside the coating during service, induce cracks and shorten the thermal cycle life of the coating.
Hot information

