

A flammable gas (such as acetylene C2H2) burns in premixed air or oxygen to form a flame.
In the flame combustion reaction, there are primary combustion and secondary combustion problems. The primary combustion reaction of an oxy-acetylene flame is
C2H2 +O2 = H2 +2CO (3-6)
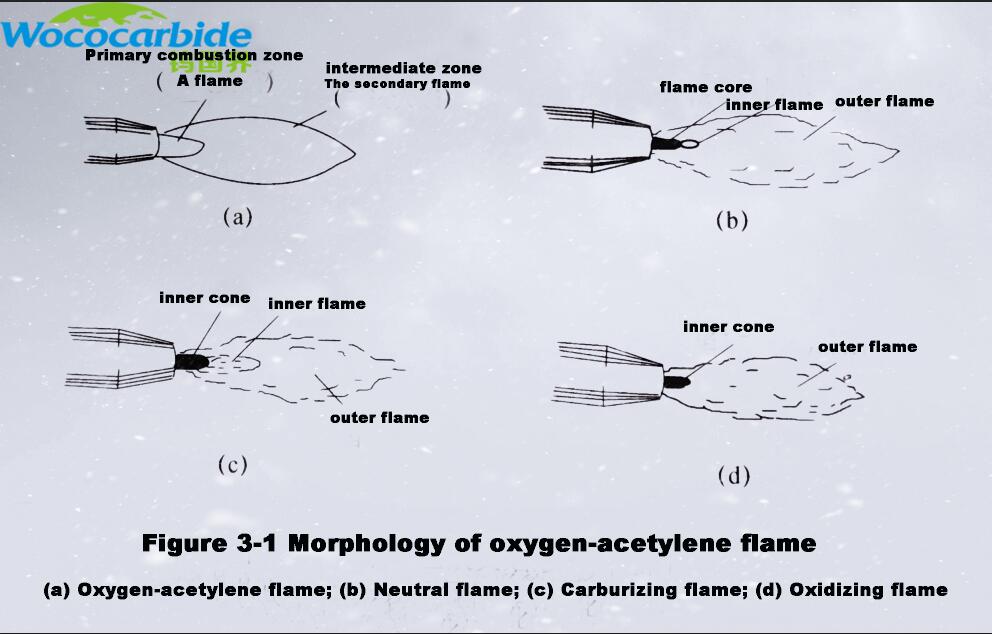
The flame formed by the primary combustion reaction is the flame core in the oxy-acetylene flame (the conical and shiny part near the torch nozzle hole), as shown in Figure 3-1(a). Of course, the combustion of oxygen and acetylene does not completely burn off all the combustibles, and the primary combustion products can also be burned. Therefore, secondary combustion will also occur, the reaction of which is
2CO + O2 = 2CO2 (3-7)
H2+O2 = H2O (3-8)
Secondary combustion is the secondary combustion of the intermediate products of primary combustion with oxygen in the surrounding air to generate stable final products. The flame formed by the secondary combustion is the outer flame in the oxy-acetylene flame. (Figure 3-1(a))
When the ratio between fuel gas (C2H2) and auxiliary gas (O2) is adjusted and controlled, oxygen-acetylene flames with three morphologies can be obtained, which are suitable for spraying of different materials. These three morphologies are neutral flame (Fig. 3-1(b)), carbonized flame (Fig. 3-1(c)), and oxidizing flame (Fig. 3-1(d)).
When the mixing ratio of oxygen and acetylene is 1.0~1.2, a neutral flame is formed (Fig. 3-1(b)), which neither oxidizes nor reduces the molten material. The neutral flame can be divided into three parts: the flame core, the inner flame and the outer flame. The flame core is sharp cone-shaped, bright white, and has a clear outline; the inner flame is blue-white, the outline is unclear, and there is no obvious boundary with the outer flame; the outer flame gradually changes from lavender to orange-yellow from the inside to the outside. There is essentially no free oxygen and free carbon in the primary flame.
When the mixing ratio of oxygen and acetylene is less than 1.0, the resulting flame is a carbonization flame (Fig. 3-1(c)), also known as a reducing flame, which is a flame with reducing and carbonizing effects. Because the oxygen is not enough to completely burn the acetylene, the excess acetylene is decomposed into carbon and hydrogen, and the carbon will penetrate into the molten material to increase the carbonization of the material, so it is called a carbonization flame. The flame structure is also divided into three parts: the flame core, the inner flame and the outer flame. The flame core is white and the periphery is slightly blue; the inner flame is pale white, and the outer flame is orange-yellow. When the amount of acetylene is large, it also brings black smoke, and the flame is long and soft.
When the mixing ratio of oxygen and acetylene is greater than 1.2 and burns, an oxidizing flame is formed (Fig. 3-1(d)). The structure of the oxidizing flame can be divided into two parts: the flame core and the outer flame. There is excess oxygen in the flame, and an oxidative oxygen-rich zone should be formed outside the flame core. The flame core is short and sharp, bluish-white; the outer flame of the flame core is a slightly purple outer flame, which is shorter than the normal outer flame and the flame is straight.
Hot information

