

3.2 Coating bonding mode
The combination of the coating metal and the base metal, no matter in the process of metal ion deposition, or in the subsequent possible artificial aging or natural aging, this lattice structure always exists in some form that is conducive to the reduction of system energy, which can make the coating Obtain metal bonds that are much stronger than mechanical adhesion. Due to the difference of the plating seed metal and the growth conditions of the plating layer, the crystal structure and lattice constant of the two bonded metals may be different, and the lattice structure obtained by the plating layer with good bonding shows different bonding modes and matching relationships.
The simplest form of bonding mode is that the coating is deposited on the same metal substrate. Under suitable conditions, the coating metal maintains the original orientation epitaxial growth of the lattice on the base metal, and the epitaxy of the base structure may reach 4000 nm or thicker. degree. In this case, the bonding force is the cohesion of the metal, and the coating can obtain the desired bonding strength.
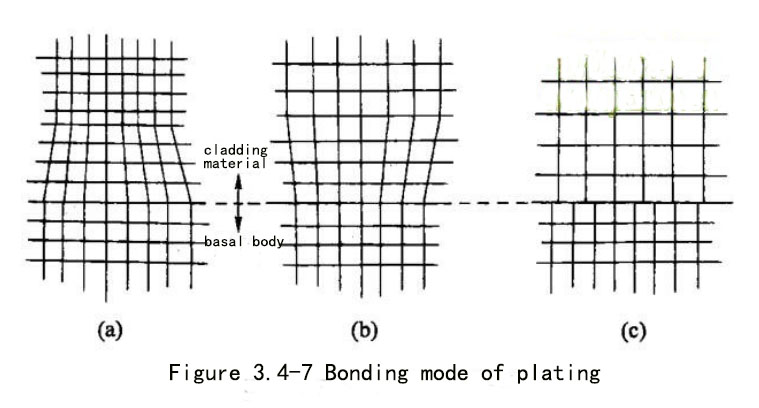
Most of the two sides of the actual coating are different metals, and even some are alloys, and the crystal structure and lattice constants are different. In order to achieve a firm bond, the crystal structure of the metals on both sides of the bond must be the same (or similar), and the lattice constants are similar. Even if their crystal structures and lattice constants are different, if the lattice spacing is not too different, a strong bond like the same metal can be achieved. This binding relationship can be represented by the binding mode diagram shown in Figure 3.4-7a or b. That is, at the beginning of plating, the electrocrystallization process tends to maintain the original orientation of the lattice on the substrate, and the epitaxial growth of the new phase along the substrate structure may still reach a considerable thickness (100-500mm). Parallelism of lattice orientation occurs when the lattice spacing in the parallel direction does not exceed 15%. At this time, according to the different formation conditions of the metal bonding layer, the coating metal will temporarily expand or contract according to the lattice constant of the base metal; The initial metal coating adapts to the lattice distortion of the matrix metal, and under certain process conditions, it will also lead to the transitional adaptation strain of the matrix metal lattice to the coating lattice at the junction surface. For example, copper and nickel are both of the same face-centered cubic lattice type, the lattice constants are 0.361 run and 0.352 nm, respectively, and the lattice constants differ by only 2.5%, which can achieve close bonding and are commonly used combined coatings; iron and chromium are also Both have the same body-centered cubic lattice, with lattice constants of 0.287 nm and 0.288 nm, which are almost equal, guaranteeing good bonding even if both are hard metals.
When the lattice types of the coating and the substrate are different, or their lattice parameters are quite different, new crystals (nuclei) will be generated, and the crystal structure will vary according to the plating conditions and the inherent crystallization of the metal to be plated, It will grow along the crystallographic direction of the base metal to form an initial bonding layer. As shown in Figure 3.4-7c.
If the crystalline structure of the deposited layer and the matrix material are completely different, in practice a certain degree of epitaxial growth often occurs under optimum conditions. Generally speaking, the orientation of the deposited structure is often an attempt to obtain the best coordination of the atoms deposited by the discharge. The optimal deposition conditions are generally: the surface layer structure of the substrate has large grains, the electrodeposition process parameters are favorable for lateral growth, and the surface layer structure of the substrate is fully exposed. From the characteristics of the electrocrystallization process, the movement of particles (including ions to be discharged and adatoms) along the interface between the cathode and the solution is required to be freer, that is, mass transfer or particle migration is more convenient, and ions on the interface are required. The concentration is higher. In this way, the control of the deposition growth by the surface structure of the substrate will be carried out under the conditions of lower current density, higher bath concentration, higher bath temperature, and less agitation, and it is easy to form the epitaxial orientation of the deposition layer structure.
In actual production, when the surface of the substrate is mechanically polished or a large amount of surface active additives are added, the influence of the surface structure of the substrate on the growth of the deposited layer will disappear quickly during the electroplating process, and the main factors affecting the structure of the coating will be concentrated. In terms of the bath formula, the actual state of the bath and the process parameters of the electroplating process. Therefore, increasing the current density, increasing the polarization, or adding organic additives, etc., often reduces the degree of epitaxy.
Under normal circumstances, with the thickening of the deposition layer, the coating structure starts from epitaxy, and after a transition stage, it turns to its own structural mode, showing the preferred orientation characteristics of the coating determined by the bath and process parameters. After the coating continues to grow to a certain thickness, it often forms facets and develops special textures. Under some conditions, the crystal morphology develops as agglomerated form of facets, and the outer surface of the coating will become rougher as the coating thickness increases.
For the transition and disappearance of the joint interface of the coating, which continues the transition and disappearance of the strain on both sides, and enables the coating to obtain a good combination of thin layers, we call it a tight bonding layer. Obviously, it includes the possible strain region of the base metal on the bonding surface and the epitaxial growth region of the coating, as well as the possible matching growth region and the alloyed region formed by the interdiffusion of atoms at the bonding interface. In principle, as long as the lattice on both sides of the bonding interface can reach the distance between atoms and metal bonding occurs, such a tight bonding layer exists. Only when the compact layer reaches a certain macroscopic thickness (such as more than 100 u n) and surface coverage, does it show its contribution to good bonding. Many actual coatings are within the range of general metallographic photos, and no obvious close-bonded layer characteristics are observed, but they can also have satisfactory bonding strength, which may be due to the close coating of the crystal planes on both sides. Although there is a large dislocation density, which weakens or reduces the metal bonding force between some lattice atoms, the overall bonding force is still strong metal bonding force. If this metal bond force is damaged, it may be lower than the cohesion force of the metal body phase on both sides of the bonding surface, or higher than the cohesion force of one of the metal materials, which is related to the plating seed and electroplating process.
When two or more atoms are co-deposited to form an alloy coating, the crystal structure depends on the atomic structure of the constituent elements and the coating formation process. Generally, when the cohesion between atoms of the same species is greater, a eutectic alloy coating will be formed; if the force between atoms of different species is greater, a mixed-type (solid solution) alloy coating will be formed. The obtained alloy coating may have a microcrystalline or amorphous structure, and if it is crystallized by heating, an intermetallic compound will be precipitated to become a eutectic alloy coating. In the metal bonding of the alloy coating, there may also be a tight bonding layer formed by the continuous strain of the base metal surface and the alloy coating. For example, the preferential deposition of some alloy components has been observed during the study of the initial growth stage of alloy coatings. In a word, the alloying of the coating is beneficial to change the crystal structure and lattice parameters, adjust the mismatch of dislocations, and achieve a good combination of the coating and the substrate.
Hot information

